MMS versus CDS
To gain a comprehensive understanding of the distinctions that exist between chlorine dioxide (ClO₂) gas and a mixture of sodium chlorite (NaClO₂) combined with an acid, which is commonly referred to as MMS (Miracle Mineral Solution) or CDH (Chlorine Dioxide long term mix), it is crucial to delve into the underlying chemistry. This includes examining the specific reactions that take place when these substances are mixed, as well as the various compounds that are produced as a result of these reactions.
When sodium chlorite is combined with an acid, a chemical reaction occurs that generates chlorine dioxide gas. This reaction can be represented by a simplified equation illustrating how the acid facilitates the conversion of sodium chlorite into ClO₂. The resulting chlorine dioxide is an efficient oxidizing agent, which means it has the ability to interact with and neutralize various pathogens, toxins, and other harmful substances in the body.
In contrast, when chlorine dioxide is used in its gaseous form, without the presence of an acid or sodium chlorite, it acts directly on cells. The mechanism by which ClO₂ operates involves its interaction with the electromagnetic charges present in biological systems. This interaction can enhance cellular function and energy levels, allowing compromised or energy-depleted cells to regain their normal operation.
The differences in the manner of application and the chemical processes involved highlight the significance of understanding these two forms of chlorine dioxide. Each has its own unique properties, applications, and potential benefits for therapeutic use, especially in the realm of electromolecular medicine.
Thus, by thoroughly examining the chemistry of chlorine dioxide gas alongside its mixtures with sodium chlorite and acid, we can appreciate not only their individual characteristics but also their implications for medical treatment and health improvement. This understanding is pivotal for medical professionals who are interested in exploring innovative therapeutic approaches based on the principles of electromolecular medicine.

Chlorine Dioxide (ClO₂)
Chlorine dioxide is a stable molecule that can be dissolved in water to form a solution known as CDS (Chlorine Dioxide Solution). The molecular structure of ClO₂ is:
ClO2
In solution, ClO₂ can exist in equilibrium, but it maintains its integrity in a stable form without undergoing continuous reactions unless it interacts with other substances.
Sodium Chlorite (NaClO₂) and Acid Reaction
When sodium chlorite (NaClO₂) is mixed with an acid (e.g., hydrochloric acid, HCl), it undergoes a chemical reaction that produces chlorine dioxide (ClO₂) and sodium chloride (NaCl). The reaction can be represented as follows:
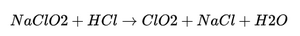
This reaction generates chlorine dioxide, but also results in the presence of residual sodium chlorite in the solution. The presence of acid lowers the pH of the solution and creates a dynamic equilibrium that can lead to ongoing reactions.
Stability and pH Considerations
1. Chlorine Dioxide Stability: In a CDS solution, ClO₂ remains stable and does not react further unless conditions are altered (e.g., introduction of strong oxidizers or extreme pH changes).
2. Sodium Chlorite in Acid: When NaClO₂ is dissolved in an acidic environment, it can continuously produce ClO₂ until all sodium chlorite has reacted. The pH of such a solution is typically less than 7, indicating an acidic environment.
The ongoing presence of sodium chlorite means that even as ClO₂ is generated, residual chlorite ions remain in the solution, potentially leading to further reactions depending on concentrations and conditions:
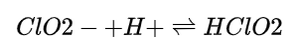
The equilibrium involving chlorite ions and hydrogen ions can shift based on the pH, leading to variations in the concentration of both chlorine dioxide and chlorite.
Summary of Key Differences
- Molecular Integrity: CDS maintains the integrity of ClO₂ without residual reactants or ongoing reactions, while a mixture of NaClO₂ and acid leads to continuous generation of ClO₂ until all chlorite reacts.
- pH Impact: The pH of a CDS solution remains neutral (close to 7), while a NaClO₂ and acid mixture results in a much lower pH due to the presence of free hydrogen ions from the acid.
- Chemical Behavior: CDS exhibits stable therapeutic properties without ongoing reactions, whereas the NaClO₂ mixture is subject to chemical changes that can affect its efficacy.
Conclusion
The fundamental differences between CDS and a mixture of sodium chlorite with acid lie in their chemical stability and behavior in solution. Understanding these differences is crucial for appreciating the therapeutic potential of CDS in electromolecular medicine. The therapeutical use of MMS or CDH theirfore is not longer supported.
