How many Oxygen molecules are in a CDS protocol?
by Dr.h.c. Andreas Ludwig Kalcker
We know that CDS, or Chlorine Dioxide Solution, liberates oxygen in the bloodstream, but how much oxygen is actually released? Is it truly significant for our health and well-being? Although this inquiry is primarily a mathematical calculation, exploring these figures might aid in understanding the remarkable healing phenomena we are observing with the use of CDS. The impact of oxygen liberation on various bodily functions and healing processes can provide valuable insights into its effectiveness and potential benefits. This exploration could lead to a deeper comprehension of the underlying mechanisms at play when using CDS in therapeutic applications.
How many oxygen molecules are in a CDS protocol C?
Definition of weight percent
Weight percent means that the weight of chlorine dioxide (ClO₂) is expressed relative to the total weight of the solution.
A concentration of 0.3% means that 0.3% of the total solution’s weight is ClO₂.
Calculate the mass of ClO₂ in 10 ml 0.3% solution
Suppose you have 1 liter of solution, and the density of the solution is roughly the same as water (1 g/ml), so 1 liter ≈ 1000 grams:
- Total weight of the solution = 1000 g
- Concentration = 0.003 (0.3%) or (3000ppm)
To calculate the weight of ClO₂:
Weight of ClO₂=0.003×1000g=3g
So, in 1 liter of this 0.3% solution, there are 3 grams of ClO₂.
If the total volume of the solution changes (e.g., 10 ml etc.), you would adjust the total weight accordingly and use the same formula.
- For 10 ml of solution (≈ 10 g):
Weight of ClO₂=0.003×10g=30mg
Note: Solution density
If the density of the solution differs from that of water, you will need to adjust the total weight accordingly. In most cases, the density of water (1 g/ml) is used as a reasonable approximation unless the difference is significant.
Convert mass to grams
Convert 30 mg to grams:
30 mg=30g / 1000=0.030 g
Calculate moles of ClO₂
The molar mass of ClO₂:
- Chlorine (Cl) = 35.453 g/mol
- Oxygen (O) = 16.00 g/mol
- Molar mass of ClO₂ = 35.453 g/mol + 2×16.00 g/mol = 67.453 g/mol
Mole of ClO₂=0.030 g /67.453 g/mol≈0.000444 mol
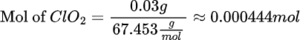
Determine the number of oxygen molecules
Each molecule of ClO₂ contains one oxygen O2 molecule, so:
Mol of O2=0.000444 mol
Now, using Avogadro's number
6.022 x 1023 molecules/mol
to find the number of oxygen molecules:
Number of O2 molecules=0.000444 mol×6.022×1023 molecules/mol
this gives: ≈2.678×1020 molecules
Average number of red blood cells in the body
The number of red blood cells (RBCs) in blood can vary, but on average, a healthy adult has about
4.5 to 5.5 million red blood cells per microliter (µl) of blood.
To calculate the total number of red blood cells in 5 liters of blood (everage healthy adult blood volume), follow these steps:
Convert liters to microliters:
There are 1,000,000 microliters (µl) in 1 liter, so in 5 liters:
5 liters=5,000,000µl
Calculate the total number of red blood cells:
Using an average of 5 million RBCs per µl:
Total RBCs=5,000,000µl×5,000,000RBCs/µl
Total RBCs=25×1012RBCs
So, in 5 liters of blood, there are approximately 25 trillion 25 x 1012red blood cells.
Calculate the number of oxygen molecules per red blood cell
Let's calculate the number of oxygen molecules per red blood cell.
Given:
- Total number of oxygen molecules: 2.678×1020 O₂ molecules.
- Total number of red blood cells: From the previous calculation, about 25×1012 RBCs (25 trillion red blood cells).
To find how many oxygen molecules we have for each red blood cell, divide the total number of oxygen molecules by the total number of red blood cells:
Oxygen molecules per RBC= 2.678∗1020 O2 molecules / 25∗1012 RBCs= 10,712,000 O2 molecules / RBC
Result:
For each red blood cell are approximately 10.7×106 oxygen molecules (about 10.7 million oxygen molecules) available .
