CDS mechanism of action
Chlorine dioxide interacts with sulfur-containing compounds found in bacteria, rendering them unable to reproduce. Additionally, it exhibits antifungal properties by reacting with ergosterol in fungal cell walls, converting it into ergocalciferol (Vitamin D₂) through a process that involves the cleavage of the B-ring. Research has also demonstrated its effectiveness against various viruses, including hepatitis A, B, and C, as it diminishes viral replication in the lungs.
Chlorine dioxide (ClO₂) operates through a distinct mechanism compared to other disinfectants. When introduced into water, it dissolves as an ion, generating a negative charge around the water molecule, particularly in the presence of salts. This negative charge effectively neutralizes the positively charged proteins of viruses, leading to their deactivation. Furthermore, chlorine dioxide is capable of penetrating the outer shell of encapsulated viruses as a gas.
Chlorine dioxide is available in various forms, which can yield different outcomes. In 2012, Jim Humble referred to the gas dissolved in water as CDS. Unlike MMS, which combines sodium chlorite (NaClO₂) with an acid and can lead to stomach upset and other side effects, CDS does not contain sodium chlorite and therefore does not generate any by-products when dissolved in water. The primary advantage of using chlorine dioxide in its CDS form over chlorine lies in its neutral pH and safety for human health and the environment. Unlike chlorine, chlorine dioxide does not produce harmful trihalomethanes (THMs).
While inhaling large amounts of the gas should be avoided, toxicological studies indicate that CDS can be safely administered through oral, intravenous, buccal (into the oral mucosa), transdermal (through the skin), or direct topical applications. It proves effective in treating bacterial and viral infections, making it safe for use at appropriate dosages.
In a venous blood gas analysis, the following findings were noted:
- The blood pH became more alkaline, indicating a reduction in acidity and an increase in basicity.
- Blood oxygen levels increased, suggesting enhanced oxygenation throughout the body.
- The concentration of carbon dioxide (CO₂) in blood decreased, implying effective CO₂ elimination via respiration.
- There was an observable improvement in acid-base balance, particularly in base deficit, reflecting better pH regulation within the body.
- Blood glucose levels normalized, with reductions in hyperglycemia noted in other cases.
- A significant decrease in blood lactic acid levels was observed, indicating improved removal of metabolic waste products.
Overall, the venous blood gas results reflect improvements across crucial areas for the organism's proper functioning, including acid-base balance, oxygenation, metabolic activity, and blood glucose regulation.
Images depicting the effects of CDS on blood:
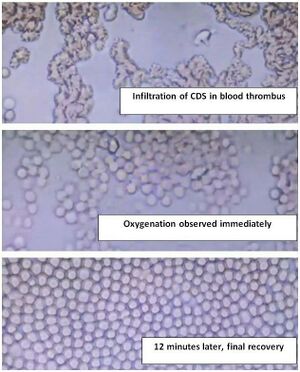
https://dioxitube.com/w/3yVQD2hkXDDTPTZbxUDLJA
In these phase contrast microscopy images, the impact of CDS on small red blood cells is clearly visible. Initially, the cells were highly agglutinated and oxygen-deprived. Following the infusion of CDS at a maximum concentration of 3000 ppm from the left side, immediate signs of oxygenation can be observed. After a mere 12 minutes, all blood cells exhibit optimal oxygenation levels.
