Covid vaccine induced rash in young girl
COVID VAX INJURY SKIN RASHES CURED IN ONE MONTH WITH THE UNIVERSAL ANTIDOTE (CHLORINE DIOXIDE)
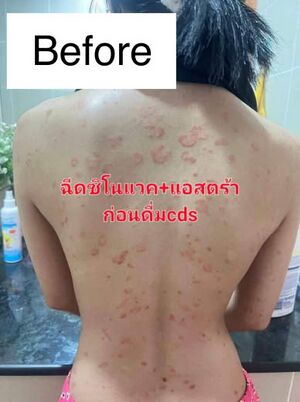
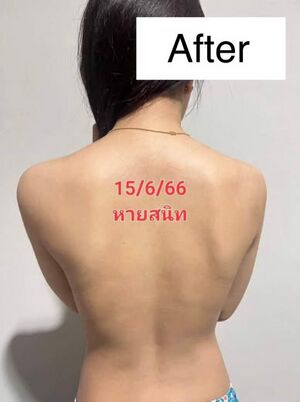
Case Study: Resolution of Severe Post-Vaccination Generalized Itchy Rashes Using Chlorine Dioxide Solution (CDS) Protocol
Patient Background and Clinical Presentation
A female patient developed extensive itchy rashes covering her entire body following administration of two COVID-19 vaccine doses: the first dose from Sinovac and the second from AstraZeneca. The onset of dermatological symptoms occurred shortly after vaccination, manifesting as widespread pruritic eruptions resistant to standard treatments.
Prior Treatment Attempts
Initial management consisted of topical corticosteroids aimed at reducing local inflammation and systemic immunosuppressive medications intended to modulate the immune response. Despite these interventions, the patient’s condition showed no improvement, and the rash continued to persist and spread. This clinical scenario suggested a treatment-resistant immune or inflammatory reaction possibly linked to vaccine-induced immune activation.
Initiation of CDS Protocol
Given the lack of response to conventional therapies, the patient opted to begin treatment with the Chlorine Dioxide Solution (CDS) protocol, a novel electromolecular therapy designed to restore cellular charge balance and improve cellular energy metabolism, thereby enhancing immune regulation and detoxification.
The CDS administration regimen was as follows:
- Day 1: 10 ml of CDS diluted in 1 liter of water, ingested throughout the day.
- Days 2 through 6: Increased daily dosage to 20 ml of CDS in 1 liter of water.
- Weeks 2 to 4: Further escalation to 30 ml of CDS daily, consistently maintained for approximately three weeks.
- Week 5 onward: Dosage increased to 40 ml daily, maintained for the remainder of treatment.
Throughout this period, the patient monitored her symptoms carefully and noted transient Herxheimer reactions—a well-documented process characterized by temporary worsening or emergence of symptoms during detoxification, reflecting the body's response to microbial die-off or toxin release.
Therapeutic Response and Outcome
The patient reported gradual improvement starting within the first two weeks of CDS therapy. The intensity and extent of the itchy rashes decreased progressively. By the end of approximately one month from the initiation of CDS treatment, she achieved full remission of skin symptoms with no residual itching or rash visible.
No adverse effects related to CDS were reported beyond mild Herxheimer reactions, which subsided over time with continued treatment.
Discussion
This case illustrates a successful application of CDS in managing a complex dermatological reaction refractory to conventional immunosuppressive therapy post-COVID-19 vaccination. The underlying mechanism involves CDS’s ability to restore cellular electromagnetic charge balance and enhance mitochondrial function, facilitating cellular detoxification and immune modulation. The sequential dosage increase allowed for safe adaptation and maximized therapeutic benefits while monitoring for detoxification responses.
Herxheimer reactions observed during treatment are consistent with activation of endogenous cleansing pathways, supporting the hypothesis that CDS promotes removal of inflammatory mediators and toxins contributing to skin pathology.
Conclusion
The CDS protocol demonstrated significant clinical efficacy in resolving severe generalized itchy rashes unresponsive to standard steroid and immunosuppressive treatments in a post-vaccinated patient. This case supports further clinical evaluation of CDS as a complementary or alternative therapy in immune-mediated dermatological conditions, especially those associated with vaccine reactions or chronic inflammation.
