CDS reaction in the body
In contrast to the belief held by many regarding the behavior of ClO2, it is essential to understand that chlorine dioxide does not simply and directly convert into chlorine and oxygen. Instead, this process involves various intermediate steps that are contingent upon specific environmental conditions and factors. These intermediary reactions can significantly influence the pathway and kinetics of the conversion, indicating a more complex mechanism at play.
The dissociation process of chlorine dioxide (ClO₂) to hypochlorous acid (HClO) occurs in several steps, which are mainly influenced by chemical reactions and environmental conditions. Chlorine dioxide is a strong oxidizing agent with exceptions to higher ORP substances like OH radicals Hidroxils .
Chlorine dioxide can be dissolved in water, where it is partially converted to hypochlorous acid under certain conditions. The general process can be represented as follows:
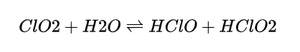
In this step, chlorine dioxide is hydrolyzed to form hypochlorous acid and chlorous acid (HClO₂). Hypochlorous acid is an important disinfectant and plays a role in the body's immune system.
The conversion of chlorine dioxide (ClO₂) to hypochlorous acid (HClO) involves a series of chemical reactions influenced by various environmental factors. Below is a detailed explanation of this dissociation process, highlighting the necessary conditions for each step.
Dissociation Process of Chlorine Dioxide to Hypochlorous Acid
- Dissolution in Water:
- When chlorine dioxide is dissolved in water, it does not fully dissociate. Instead, it exists in equilibrium with its different forms. The solubility of ClO₂ in water is crucial, as it affects the concentration of the species present in solution.
- Formation of Hypochlorous Acid:
- Under acidic or neutral pH conditions, ClO₂ can undergo a reaction with water that leads to the formation of hypochlorous acid (HClO). The general reaction can be represented as:
- This reaction is favored in environments with lower concentrations of hydroxyl ions (OH⁻), which can shift the equilibrium towards the formation of HClO.
- Influence of pH:
- The pH of the solution plays a significant role in determining the extent of ClO₂ conversion to HClO. Lower pH conditions (more acidic) promote the formation of hypochlorous acid. Conversely, at higher pH levels, the formation of chlorite (ClO₂⁻) and other species becomes more favorable.
- Temperature:
- Temperature can affect the kinetics of the reaction. Higher temperatures generally increase reaction rates, potentially leading to faster conversion of ClO₂ to HClO, but may also increase the likelihood of side reactions that can produce other chlorinated compounds.
- Concentration:
- The concentration of ClO₂ in the solution is another critical factor. Higher concentrations may lead to increased reaction rates but could also result in more complex chemistry due to the formation of multiple chlorinated species.
2. Formation of hypochlorous acid
The hypochlorous acid itself can further dissociate in aqueous solution:
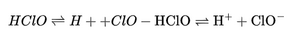
This reaction is important for antimicrobial activity, since the ClO⁻ ion plays a crucial role in combating pathogens.
3. Interaction of NaCl and O₂ in the body
Sodium chloride (NaCl) and oxygen (O₂) are essential components in the human body. Sodium chloride is often ingested through food and is important for numerous physiological processes. In the body, NaCl is dissociated into sodium (Na⁺) and chloride ions (Cl⁻).
Oxygen is essential for cellular respiration because it serves as an electron acceptor in oxidative phosphorylation, a process that produces ATP (adenosine triphosphate) for energy. The reaction can be simplified as:
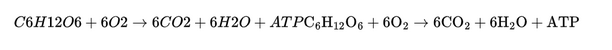
Sodium chloride can also regulate osmolarity and electrolyte balance in the body, which is crucial for maintaining fluid balance and nerve and muscle function.
Overall, the conversion processes from ClO₂ to hypochlorous acid and the interactions of NaCl and O₂ in the body are complex biochemical processes that are essential for maintaining health and physiological functions.
